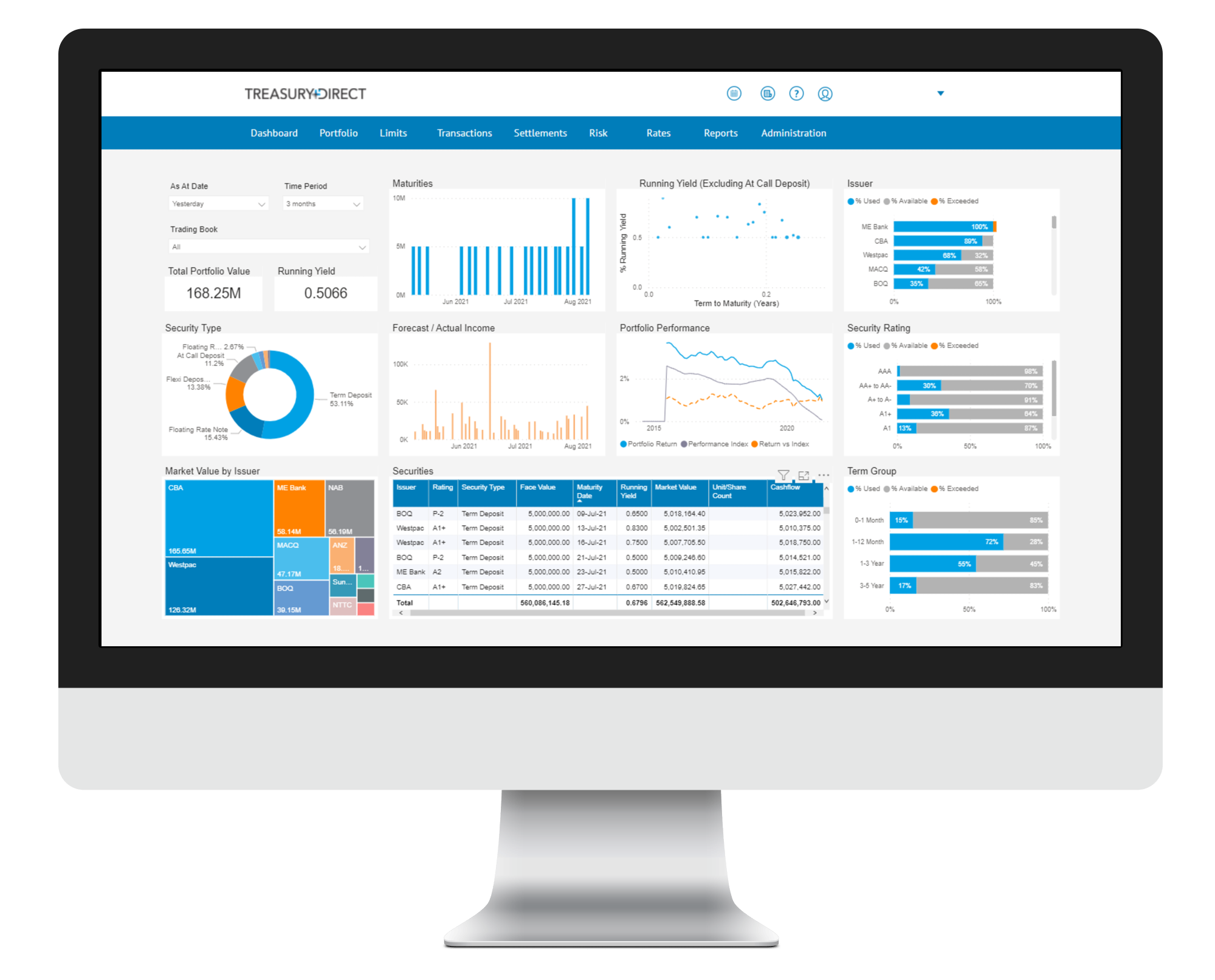
Markets Overview
- ASX SPI 200 futures down 0.3% to 7,344.00
- Dow Average little changed at 36,292.71
- Aussie little changed at 0.7290 per US$
- U.S. 10-year yield fell 3.6bps to 1.7076%
- Australia 3-year bond yield rose 2bps to 1.18%
- Australia 10-year bond yield rose 0.9bps to 1.86%
- Gold spot down 0.3% to $1,821.11
- Brent futures down 0.6% to $84.12/bbl
Economic Events
- 11am: (AU) Australia to Sell A$1 Billion 0.25% 2025 Bonds
- 11:30am: (AU) Nov. Investor Loan Value MoM, est. 8.0%, prior 1.1%
- 11:30am: (AU) Nov. Home Loans Value MoM, est. 0.4%, prior -2.5%
- 11:30am: (AU) Nov. Owner-Occupier Loan Value MoM, est. 3.0%, prior -4.1%
Australian transport and freight workers who are close contacts of those infected with Covid-19 will no longer be required to isolate in an attempt to end supermarket shortages across the country. Qantas is cutting domestic and international flight capacity as the omicron coronavirus variant sweeps through Australia and across the globe, reversing an expected pickup in travel.
Other News
Hundreds of millions of people have gotten the Pfizer Inc.-BioNTech SE and Moderna Inc. Covid-19 vaccine shots. But how many know that the Pfizer vaccine is called Comirnaty? And Moderna’s? Spikevax.
Despite a year of wall-to-wall media coverage and debate, the names of the world’s two biggest Covid-19 vaccines are nowhere close to the name recognition of such products as Tylenol, Kleenex or the iPhone.
One reason is that naming requirements set up by the Food and Drug Administration and international health regulators are so complex that settling on what to call a new prescription drug — Covid-19-era aside — usually takes about two years. Few turn out as catchy as Oreos.
The regulatory goal is to make sure patients receive only the drugs prescribed to them. So a new brand name can’t be too similar to an existing one. It can neither mention the drug’s chemical components nor violate any trademark. And it must steer clear of unintended meanings in other languages.
Drugs receiving FDA approval have unique names but are getting hard to pronounce: the rheumatoid arthritis therapy Xeljanz; the prostate cancer treatment Orgovyx; and Evrysdi for spinal muscular atrophy, to name a few.
The letter y is becoming one of the most common vowels in drug names because it provides “a very stark visual differentiation,” said Scott Piergrossi, president of creative at the Brand Institute. The Miami-based company was hired to name the Moderna and Pfizer-BioNTech vaccines, as well as several Covid-19 treatments.
In April 2020, a team from the Brand Institute began working on vaccine names. BioNTech, which developed the vaccine with Pfizer, wanted one to reflect both a sense of community and its groundbreaking use of messenger RNA, the molecular couriers that deliver genetic instructions.
But mRNA sounded a lot like Mirena, the name of a birth control device. It also echoed Moderna, which was developing a rival Covid-19 vaccine.
“Starting off with an mRNA combination could have been a lost cause,” Mr. Piergrossi said. The firm spent about six months sifting through a thousand or so candidates. Among them: Rnaxcovi, Kovimerna, Covuity and Comirnaty, the eventual winner.
The Brand Institute uses machine-learning-based computer models to make sure a name is at least 70% dissimilar from another, Mr. Piergrossi said. (The 70% standard was set by an FDA process called the Phonetic and Orthographic Computer Analysis.)
The company screened prospective Moderna and Pfizer-BioNTech vaccine names against the FDA’s database of 36,000 existing names and 90,000 in the European Medicines Agency database. It also checked tens of thousands of words in languages spoken throughout international markets to make sure the names would work around the world.
Brand experts said drug names often take time to catch on. The Pfizer-BioNTech and Moderna vaccines are in their early days — only Pfizer’s is fully approved by the FDA; Moderna’s has emergency-use authorization. “These names will find their place in the pharmaceutical lexicon,” Mr. Piergrossi said
Former FDA officials said the agency began instituting stricter naming guidelines for drugs and vaccines in the mid-2000s, seeking to combat prescription drug mix-ups. Above all, the drug’s name can’t look or sound like another medicine’s. The FDA conducts handwriting tests to make sure the name isn’t easily confused with another when prescriptions are being filled.
“Most people outside of the industry have the same sort of impression, ‘Who knew it was that complicated? I thought you just put up some Scrabble tiles and get yourself a name,’” said Mike Pile, managing creative director of Uppercase Branding, which has named prescription drugs.
So far, few people know or bother to use hard-won brand names for Covid-19 vaccines. “There’s already an easier and well understood name — Pfizer,” said Harry Thomas, a physician in Austin, Texas. He found the brand name, Comirnaty, confusing.
Moderna considered more literal names like Covidvax before agreeing on Spikevax, which is meant to invoke the coronavirus’s spike-shaped protein.